So I recently got engaged, but JEEZ I don’t want to go on about it, stop with all the questions!
As I stare at this new (very lovely) band of metal and stones on my finger, I naturally begin to think about the science behind it (shortly followed by the thought that I am lucky that anyone wants to spend their life with me). What makes diamonds and other gemstones so special? And what the hooters is the story behind the rather odd ritual of engagement rings in the first place?! Who cares? I DO!
History
Before we delve into the hardcore science, let us first take a quick look at the history of the engagement ring. Apparently such rings were used by the ancient Egyptians to symbolise a never-ending cycle of love. Aaww. The significance of the ring finger being the forth finger on the left hand comes from the Ancient Greeks, because they believed that this finger contained a vein which led directly to the heart. The Romans then had to come along and sort of ruin all these romantic images, as the ring for them symbolised ownership of their woman, rather than love. However, it was not until 1215 that engagement rings as we know them today, used to show loyalty and devotion to one’s lover until the date of marriage, appeared, and this was thanks to Pope Innocent III’s establishment of a waiting period between the promise of marriage and the ceremony itself. But in those days it was only the very rich who could afford such luxuries, and it wasn’t until the end of the 19th century that it became commonplace in the West to offer an engagement ring (before this, wives-to-be were traditionally offered a sewing thimble instead. I don’t want to sound like a diva but I would have been most disappointed with this). And the now-traditional norm of a diamond ring? Well, you can thank a 1930s marketing campaign by a diamond company for that: ever heard of the phrase “Diamonds are forever“? Thanks, DeBeers! More about this LIE later.
Diamond science
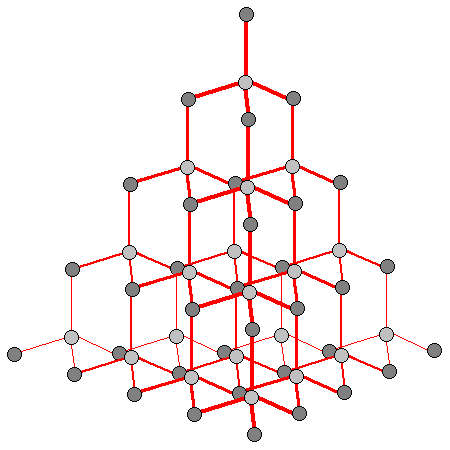
We know that diamonds are strong and shiny, but why? Diamond is a form of carbon, formed at high temperature and pressure, deep below the surface of the earth. The ones we find on the surface of the planet have come to be there due to a violent, deep-seated volcanic eruption, one of which we haven’t seen in recent times. When you look at a diamond, what you are actually looking at is a single crystal, which contains around a million billion billion atoms, perfectly arranged into a perfect pyramidal structure, like the one below.
It is this structure of diamond that accounts for the stone’s remarkable properties, such as its strength and shininess. The electrons in this structure are locked into an extremely stable state, which is what makes diamond such a famously hard and strong material (the word diamond actually comes from the Greek word for “unbreakable”). Diamonds are at the top of the Mohs scale (a scale of mineral hardness, based on the ability of one sample of matter to scratch another mineral), scoring 10/10.
But we do not only admire diamonds for their durability, they look beautiful too. This property is also down to the above crystal structure, which gives the diamond transparency and an unusually high optical dispersion. This means that as light enters the crystal, it is split into its constituent colours, giving it a gleaming rainbow sparkle.
Sturdy, strong and beautiful they may be, but one thing diamonds are not, is forever (sorry, DeBeers). All diamonds are in fact slowly (very slowly) turning into graphite (the stuff your pencils are made from), a more stable form of carbon. Don’t worry though, this process happens so slowly that our diamonds will still outlive us, by a very long way.
Rubies and Sapphires
Of course, although the diamond is still the dominant gemstone in engagement rings, many people prefer to have a splash of colour in their jewelry, which can often be provided in the form of another precious stone, often rubies and sapphires. Something I did not know before I began studying my own ring is that sapphires and rubies are in fact different coloured versions of the same mineral: Corundum. Corundum is crystalline aluminium oxide and is also extremely strong, appearing just after diamond on the Mohs scale, scoring 9. It is transparent in its natural form, but various impurities can cause it to appear a different colour, which leads to our ruby (the red variety of corundum) and sapphire (variety that encompasses all other colors, although the most popular and valued color of Sapphire is blue). The presence of element chromium is mainly responsible for the ruby-red, whereas blue sapphires exists thanks to the addition of iron and titanium to corundum.
So the next time you admire a shiny piece of jewelry, you may want to just take a second to consider its crystal structure, where the materials stand on the Mohs scale, and what elements make it up. I’m sure that’s exactly what went through Mr Loris’s head as he selected this beauty.
Sources
Miodownik, Mark (2013) Stuff Matters
http://en.wikipedia.org/wiki/Engagement_ring
http://www.gemnation.com/base?processor=getPage&pageName=forever_diamonds_1
http://www.extremescience.com/rubies.htm
http://www.mindat.org/min-1136.html